Case Studies
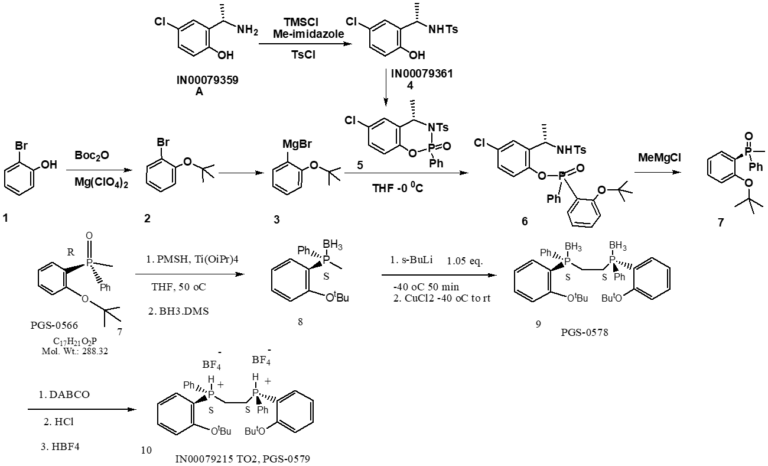
The Preparation of The Tosylate
Processing issues: The preparation of the tosylate: the protection and de-protection were used. The tBu-OPhBr formation, in the previous process, the preoxide ester and Grignard reagent were used for the transformation. The raw materials are of high cost and the conditions are unsafe. Key intermediate 6, highly flammable tBuLi was used in the old process, which was very difficult to scale-up.
Read More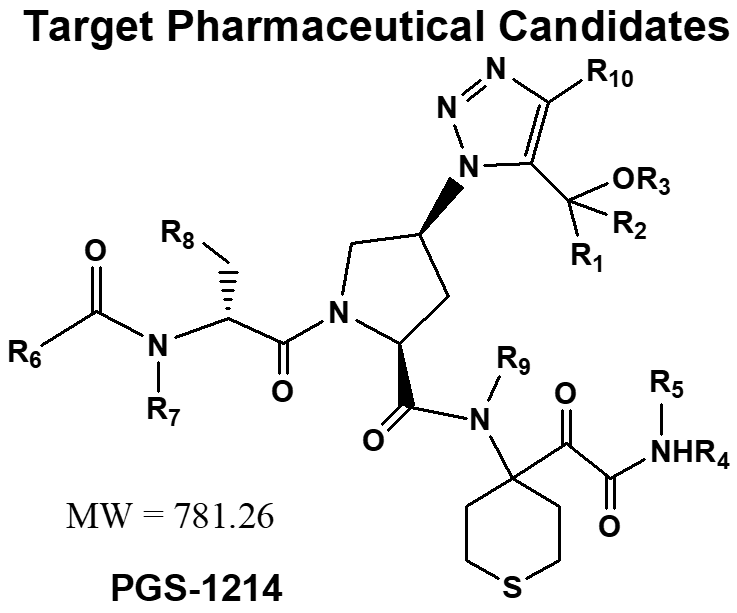
Processing and Scale-up
Processing and scale-up issues: 4 Coupling reactions, all need flash column for isolation/purification. Side-chain, tedious synthesis, difficult to make it pure. One coupling reaction using DMTMM reagent, the reaction easily to be stalled, requires adding more coupling reagent multiple time. Final API, amorphous, extremely difficult to be purified, cp only 97%. Total 9 + 2 = 11 steps, overall yield ~8%.
Read More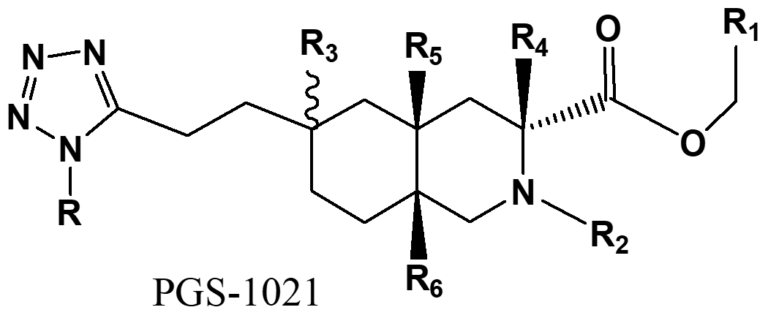
Drug Candidate for a New Indication
Processing problems: Total 12 steps with very low overall yield (<1%). Chiral resolution only had ~13% yield, 3 recrystallization. Tetrazole synthesis, lot of tar formed, difficult isolation with low yield.
Read More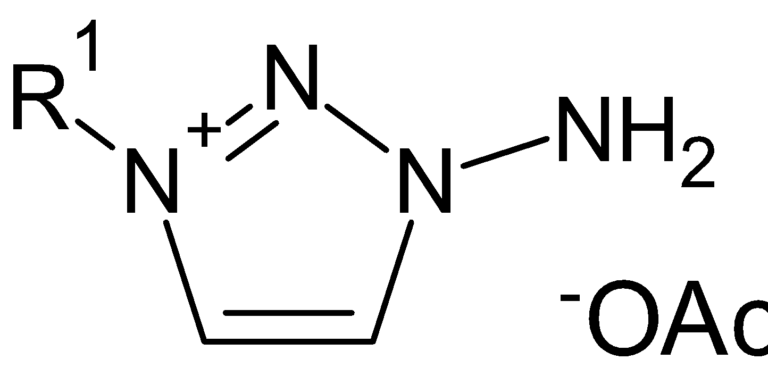
CDG 1
It involves a triazole compound. The client process involved conversion of an iodide compound into acetate salt using complicated oxidation using hydrogen peroxide and extraction of the iodine liberated.
Read More- List Item #1
- List Item #2
- List Item #3
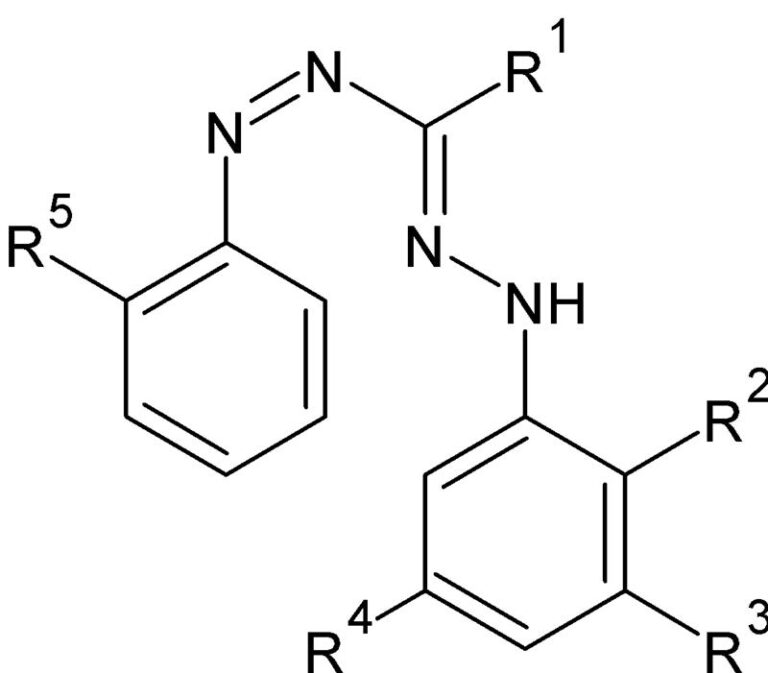
CDG 2
This involved a formazan dye used in diagnostics. The initial process involved heating up a diazonium salt to solubilize for further reaction. It is unsafe and also resulted in low yield. We modified the process by substituting DMF with water and conducting the reaction with improved yield and in a safer way.
Read More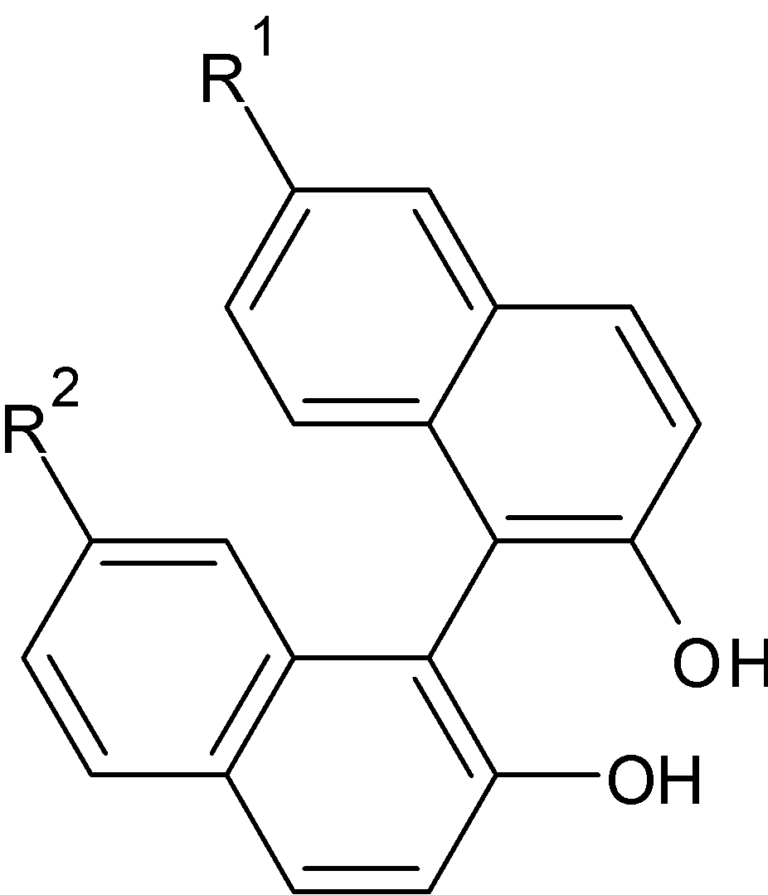
CDG 3
This involves synthesis of a binaphthol derivative. Reported methods used dilute solution and longer reaction time. We modified the reaction by mixing the starting materials as neat and conducting the reaction at a fixed temperature. Good yield were obtained by this process.
Read More